Pethidine Injection IP, 50mg/ml wholesaler retailer and distributor in Chennai, Tamilnadu

Pethidine Injection IP, 50mg/ml wholesaler retailer and distributor in Chennai, Tamilnadu
What is a Patient Information Leaflet and why is it useful?
The Patient Information Leaflet (PIL) is the leaflet included in the pack with a medicine. It is written for patients and gives information about taking or using a medicine. It is possible that the leaflet in your medicine pack may differ from this version because it may have been updated since your medicine was packaged.
Download LeafletView the patient leaflet in PDF format
Below is a text only representation of the Patient Information Leaflet. The original can be viewed in PDF format using the link above.
Pethidine Injection IP, 50mg/ml wholesaler retailer and distributor in Chennai, Tamilnadu
NRx
VERPAT
Pethidine Injection IP, 50mg/ml
DESCRIPTION
Verpat Injection is a clear, colorless liquid, filled in 2.0ml colorless glass ampoule. 50 mg/ml, 100mg/2ml Chemical name: ethyl 1-methyl-4-phenylpiperidine-4-carboxylate hydrochloride (MW 283.8). CAS Registry Number: 50-13-5.
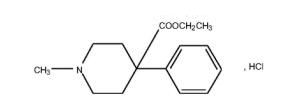
Pethidine Injection BP is a sterile, clear solution of Pethidine Hydrochloride BP in Water for Injections BP. VERPAT (Pethidine Injection BP) is available as a 50 mg/1 mL, and 100 mg/2 mL solution. The pH of the injection ranges between3.5 and 6.0.
VERPAT (Pethidine Injection BP) is available as a sterile 50 mg and 100 mg solution.
Pharmacology
Pethidine is a synthetic opioid analgesic which produces a pattern of effects similar to morphine the standard against which opioid analgesics are compared. In addition to analgesia, the effect of pethidine on the central nervous system causes respiratory depression, drowsiness, sedation, change in mood, euphoria, dysphoria, mental clouding, nausea, vomiting, and electroencephalographic changes. Large doses of pethidine may induce excitation or convulsions. Pethidine is not effective in the management of cough or diarrhoea.
As a general rule, 75 to 100 mg pethidine (parenterally) is equivalent to 10 mg morphine with respect to analgesic effect, euphoria and respiratory depression.
Pethidine has local anaesthetic activity but may be an irritant when applied locally.
Opioids exert their pharmacological actions by interaction with stereo-specific opiate receptors located in the central and peripheral nervous systems.
Pharmacokinetics
Absorption: Pethidine may be administered as an intramuscular, intravenous or subcutaneous injection. Variable absorption has been observed in some cases following intramuscular administration. It has been found that 80% or more of a 100 mg dose of pethidine administered intramuscularly is absorbed over six hours with a mean time of maximum plasma concentration at approximately 24 minutes.
Analgesia may persist for two to four hours following intramuscular, intravenous and subcutaneous administration.
Distribution: There is no specific information on the distribution of pethidine but data indicate that pethidine is extensively distributed extravascularly, primarily into rapidly perfused tissues.
The apparent volume of distribution is estimated as 4.17 L/kg. Plasma protein binding has been estimated to be 64.3%. Because of the large volume of distribution of pethidine, displacement of
pethidine from plasma proteins is not likely to cause a significant increase in free pethidine concentration.
Metabolism: Pethidine is extensively metabolised in the liver. It undergoes transformation primarily by hydrolysis to pethidinic acid, followed by partial conjugation with glucuronic acid. Pethidine also undergoes N-demethylation to norpethidine, followed by hydrolysis and partial conjugation.
Norpethidine, a major metabolite, is estimated to be half as active as pethidine as an analgesic but twice as potent as a convulsive agent as pethidine.
Accumulation of pethidine can occur in patients with hepatic dysfunction. Adjustment in dose, frequency and/or duration of pethidine therapy is recommended.
Excretion: The mean plasma clearance of pethidine following IV injection is 1.06 L/min (range 0.71 to 1.32) but only 3.8% of the dose is excreted unchanged (range 2.2 to 6.9%). Plasma clearance is significantly reduced with hepatic dysfunction.
Elimination half-life of pethidine has been estimated to be approximately 3.5 hours. This can be prolonged to 7-11 hours in cirrhotic patients and patients with acute viral hepatitis, see Metabolism for advice on dosage adjustment.
In patients with impaired renal function, persons over 60 years and the neonate, the elimination half-life of norpethidine is prolonged from the normal range of 8-21 hours to longer than 30 hours which may lead to accumulation and toxic side effects such as seizures, agitation, irritability, tremors, twitches and myoclonus. The half-life of norpethidine in pregnant women averages 20.6 hours.Pethidine and norpethidine accumulate in maternal plasma following multiple doses of pethidine during labour and maximum exposure of the foetus will result because of a continued diffusion gradient from mother to foetus. Elimination of both pethidine and norpethidine is prolonged and with long drug-to-delivery intervals and/or multiple doses the levels of norpethidine in the newborn may become clinically significant.
The urinary excretion of pethidine and norpethidine may be enhanced with acidification of the urine.
INDICATIONS
Pethidine is indicated for short-term (24-36 hours) relief of moderate to severe pain. It can be given via the following routes of administration – intramuscular, subcutaneous, slow intravenous bolus injection, intravenous infusion and patient controlled analgesia (PCA).
Pethidine is indicated for administration as an anaesthetic adjunct and for obstetric analgesia.
Contraindications
Hypersensitivity to Pethidine.
Respiratory depression, or where respiratory reserve is depleted (acute bronchial asthma, chronic airway disease, severe emphysema, severe chronic bronchitis, kyphoscoliosis).
Head injury, raised intracranial pressure (apart form introducing monitoring and diagnostic problems, hypercapnia associated with respiratory depression can itself result in elevated intracranial pressure), brain tumour.
Cardiac arrythmias, especially supraventricular tachycardias, cor pulmonale. Pethidine has a vagolytic action and may produce a significant increase in the ventricular response rate.
Concurrent use of monoamine oxidase inhibitors (MAOI’s), including selegeline, or use of MAOI’s within two weeks prior. The combination of monoamine oxidase inhibitors and pethidine has caused hypotension, hypertension, excitation, rigidity, hyperpyrexia and/or convulsions and in some cases fatalities have been reported. This combination should be avoided.
Pre-eclampsia, eclampsia.
Convulsive states such as status epilepticus, tetanus and strychnine poisoning, due to the stimulatory effects of pethidine on the spinal cord.
Diabetic acidosis where there is a danger of coma.
Acute alcoholism or delirium tremens.
Severe liver disease, incipient hepatic encephalopathy.
Patients with a low platelet count, coagulation disorders or receiving anticoagulant treatment.
Continuous Intravenous Infusion: The administration of pethidine via continuous intravenous infusion in patients with renal impairment is contraindicated.
Patient-Controlled Analgesia: The administration of pethidine via patient-controlled analgesia (PCA) in young children and adults with poor cognitive function is contraindicated. The administration of pethidine via PCA in patients with renal impairment is contraindicated.
Warning
Large doses and/or rapid intravenous administration of pethidine may produce rapid onset respiratory depression, apnoea, hypotension, peripheral circulatory collapse, bradycardia (as a result of stimulation of medullary vagal nuclei) or even cardiac arrest. Pethidine should not be administered by intravenous injection unless an opioid antagonist and facilities for controlled or assisted respiration are available.
Seizures may result from prolonged exposure or high doses of pethidine due to pethidine-associated neurotoxicity (PAN). PAN is a recognised clinical entity which is mainly due to the metabolite norpethidine (see Adverse Reactions). Norpethidine concentrations are enhanced by reduction in renal excretion as in the elderly and the very young and by increased conversion of pethidine to norpethidine due to the effects of drugs such as phenobarbitone and phenytoin. Furthermore, pethidine-associated neurotoxicity is dose related, so pethidine should not be used for periods greater than 24 to 36 hours.
Because of the spasmogenic properties of pethidine on the biliary tract and sphincter of Oddi, it should be used only when necessary and then with caution in biliary colic, operations on the biliary tract and acute pancreatitis. Pethidine may render surgical exploration of the common bile duct difficult.
Decreased gastric emptying associated with pethidine may be expected to increase the risks of aspiration either associated with pethidine induced CNS depression/coma or during or after general anaesthesia, eg: a labouring patient going on the caesarean section.
Opioids may obscure the diagnosis and/or mask the clinical course of patients with head injuries or acute abdominal conditions and should not be used unless absolutely necessary in these conditions. The respiratory depressant effects of pethidine may be markedly exaggerated in the presence of head injury.
Inadvertent intra-arterial administration can produce severe necrosis and gangrene.
Opioid analgesics have abuse potential. Psychological and physical dependence may occur with repeated dosing. Pethidine should be restricted to short-term administration for the relief of severe pain not responding to non-opioid analgesics. Abrupt withdrawal of pethidine in those physically dependent may precipitate withdrawal syndrome, including convulsions.
The risk of toxic megacolon may be increased in patients with severe inflammatory bowel disease.
The use of pethidine in patient-controlled analgesia (PCA) should be reserved for short-term (24 to 36 hours) use in patients with normal renal function who have adverse reactions to morphine. Morphine is the opioid of choice for PCA.
The risk of pethidine associated neurotoxicity (PAN) is increased in a situation in which the patient may receive large doses of pethidine, as with patient-controlled analgesia (PCA) . Caution should therefore be taken in patients receiving pethidine by PCA. Frequent clinical assessment and recording of the amount of drug used is required to minimise such risks. Clinical experience suggests that patients with normal renal function receiving more than 1000 mg/24hrs pethidine are at particular risk of developing PAN. Patients receiving over 800 mg/24hrs pethidine should be usually monitored for early signs of norpethidine toxicity (eg: twitching, anxiety).
Precautions
Serious or life-threatening reactions such as respiratory depression, coma, convulsions, possibly due to elevated levels of norpethidine and hypotension have been associated with the use of pethidine. Therefore the recommendations in the Warnings and Precautions sections should be carefully observed.
Since pethidine may cause drowsiness and general impairment of coordination, ambulatory patients should be cautioned against driving or operating machinery.
Pethidine should be used with caution in patients taking other CNS depressant drugs such as hypnotics and sedatives including barbiturates and benzodiazepines, phenothiazines, and other tranquillisers, anaesthetics, alcohol and antidepressants.
Patients with severe pain may tolerate very high doses of pethidine but may exhibit respiratory depression should their pain suddenly subside.
The elderly demonstrate an increased sensitivity to opioids relative to younger patients. Reduced liver function, renal function and plasma protein binding may contribute to the elevated plasma levels found in elderly subjects.
Since pethidine is metabolised in the liver and excreted via the kidneys, the possibility of accumulation of the toxic metabolic norpethidine should be considered in patients with hepatic and/or renal impairment (see Dosage and Administration).
Reduced cardiac output may lead to reduced hepatic perfusion and diminished metabolism of pethidine leading to accumulation of pethidine with possible toxic results.
Pethidine may cause a transient rise in blood pressure and systemic vascular resistance and increased heart rate. Therefore, it is not recommended for pain relief in cardiac infarction.
Pethidine in patients with phaeochromocytoma may result in a hypertensive crisis.
In an individual physically dependent on opioids, the administration of the usual dose of an opioid antagonist will precipitate an acute withdrawal syndrome. The severity of this syndrome will depend on the degree of physical dependence and the dose of antagonist administered. The use of opioid antagonists in such individuals should be avoided if possible. If an opioid antagonist must be used to treat serious respiratory depression in the physically dependent patient, the antagonist should be administered with extreme care and only 10 to 20% of the usual initial dose administered.
Pethidine may aggravate pre- existing convulsions in patients with convulsive disorders. If dosage is escalated substantially above recommended levels because of tolerance development, convulsions may occur in individuals without a history of convulsive disorders.
In eclampsia the combination of pethidine with phenothiazines has been reported to induce recurrence of seizures rather than stopping them. Therefore, the use of pethidine in eclampsia and pre-eclampsia is not recommended. (see Contraindications).
Pethidine, while commonly used for pain relief in obstetrics, is known to pass the placenta and may cause neonatal depression, including respiratory depression. An opioid antagonist such as naloxone may be required to reverse such depression. In the neonate, pethidine is excreted and metabolised at a significantly reduced rate compared to adults.
Orthostatic hypotension has been reported in ambulatory patients administered pethidine.
Pethidine should be given with caution and the initial dose should be reduced in patients with hypothyroidism or Addison’s disease.
Pethidine should be used with caution in patients with prostatic hypertrophy or urethral stricture.
As opiate agonists may produce hyperglycaemia, this effect should be considered when diabetics require pethidine.
There are conflicting reports about the effect of pethidine on the eye. Some reports state that pethidine and its congeners produce miosis, whereas others indicate that these drugs tend to produce mydriasis or no pupillary change. Until the effects are better defined intraocular tension should be monitored in patients with glaucoma who received pethidine.
Use in Pregnancy
Category C*. Opioid analgesics may cause respiratory depression in the newborn infant. These products should therefore only be used after weighing the needs of the mother during labour against the risk to the foetus (see also Pharmacology).
Category C = Drugs which, owing to their pharmacological effects, have caused or may be suspected of causing harmful effects on the human foetus or neonate without causing malformations. These effects may be reversible.
Drug Interactions
Pethidine has been found to interact with the following drugs:
Barbiturates, chloral hydrate, benzodiazepines: Pethidine enhances the CNS depressant effects of these drugs. In addition, the combination of pethidine and phenobarbitone may reduce the analgesic effect of pethidine in part due to the increased conversion of pethidine to the toxic metabolite, norpethidine.
Phenothiazines: CNS toxicity and hypotension including respiratory depression may occur when given together. In eclampsia the combination has been reported to induce recurrence of seizures (see Precautions).
Butyrophenones: The CNS depressant effect of tranquillisers may be increased by pethidine.
Monoamine oxidase inhibitors: Excitation, sweating, rigidity, hypertension or hypotension, coma have occurred with combination. Interaction with furazolidone is not likely until it has been taken for five days. Interaction with selegiline, a MAOI Type B, has been reported as causing delirium, restlessness, sweating and rigidity.
Paracetamol: Absorption may be reduced due to delayed gastric emptying caused by pethidine.
CNS depressants (including alcohol): Depressant effects may be enhanced by pethidine.
Phenytoin: Increased metabolism of pethidine and generation of norpethidine resulting in the possibility of increased CNS effects of norpethidine and reduced analgesia.
The effects of coumarin or indandione – derivative anticoagulants may be increased.
Concurrent use with amphetamines, which have some MAO inhibiting activity is not recommended because of the risk of serious reactions similar to those reported with other MAO inhibitors.
Adverse Reactions
As with other opioid analgesics, respiratory depression is the major hazard associated with parenteral pethidine therapy. Other adverse reactions include:
More Common Reactions
Central Nervous System – Lightheadedness, dizziness, sedation, sweating, bizarre feelings, disorientation, hallucinations, psychosis. Some of these effects seem to be more prominent in ambulatory patients and those not experiencing severe pain, and may be relieved by reducing the dose slightly and lying down.
Gastrointestinal – Nausea and vomiting, constipation.
Less Common Reactions
Cardiovascular – Hypotension, vasodilation, hypertension, tachycardia, bradycardia, gangrene, following inadvertent intra-arterial administration.
Dermatological – Rash, pruritus, urticaria, erythema, injection site complications eg: local irritation and induration, fibrosis of muscle tissue with frequent repetition of intramuscular injection.
Gastrointestinal – Decreased gastric emptying.
Genito-urinary – Urinary retention and anuria.
Hepatic – Increased biliary tract pressure, choledochoduodenal sphincter spasm.
Nervous System – Pethidine associated neurotoxicity (see Warnings), or neuropsychiatric toxicity ie. auditory and visual hallucinations, irritability, agitation, hypomania, paranoia, delirium and complex partial seizures, vertigo, dizziness, coma, headache, convulsions or tremor, respiratory depression, cold clammy skin, sweating and pallor. Inadvertent injection around a nerve trunk may cause sensory-neural effects, which is usually, but not always transitory.
Psychiatric – Neuropsychiatric toxicity, hyperactivity or agitation, depression, mental clouding, dysphoria.
General – Dry mouth, weakness, hypersensitivity.
Instructions to be Given to Patient
CNS depression is increased when pethidine is co-administered with alcohol, butyrophenones, hypnotics, sedatives, phenothiazines, tricyclics, antihistamines and other CNS depressant agents.
Driving and operating dangerous machinery should not be contemplated until the day following the last dose of pethidine.
Addictive Potential
Dependence on pethidine may result after treatment with therapeutic doses. Use of pethidine should therefore be restricted to short-term administration for the relief of severe pain not responding to non-opioid analgesics. Abrupt withdrawal of pethidine in physically dependent individuals may precipitate acute withdrawal syndrome, including convulsions.
Dosage and Administration
Pethidine is indicated for short-term (24-36 hours) relief of moderate to severe pain. It can be given via the following routes of administration – intramuscular, subcutaneous, slow intravenous bolus injection, intravenous infusion and patient controlled analgesia (PCA).
An opioid antagonist and facilities for administration of oxygen and control of respiration should be immediately available during and immediately following intravenous administration of pethidine.
Adult Dosage
1. Analgesia
Dosage should be adjusted according to the severity of pain and the response of the patient and also depends on patient profile eg: age, weight, sex, previous exposure to narcotics.
25 to 100 mg by IM (preferred) or S.C. injection, every 3 to 4 hours. 25 to 50 mg slow IV injection, every 3 to 4 hours (see Warnings).
Usual dose is 200 mg/day by the IV route.
Intravenous injection should be made very slowly, preferably using a diluted solution.
For continuous intravenous infusion adequate analgesia should be established prior to commencement of the infusion. A dosage of 0.3 mg/kg/hr is recommended as the initial intravenous infusion rate.
Clinical experience suggests that patients with normal renal function receiving more than 1000 mg/24hrs pethidine are at particular risk of developing pethidine associated neurotoxicity (PAN). Patients receiving over 800 mg/24hrs pethidine should usually be monitored for early signs of norpethidine toxicity (see Warnings).
2. Obstetric Analgesia
50 to 100 mg by IM (preferred) or S.C. injection, administered when pain becomes regular. May be repeated 3 to 4 times at one to three hour intervals if necessary.
Note: Maximum of 4 doses in 24 hours
3. Anaesthesia Adjunct
As premedication, intramuscular (preferred) or subcutaneous, 50 to 100 mg thirty to ninety minutes prior to anaesthesia.
As an adjunct to anaesthesia, intravenous, by repeated slow injection of fractional doses of a solution diluted to 10 mg per mL. See Warnings prior to administering by the IV route. Dosage by this route should not exceed 25 to 50 mg.
Note: Dosage must be titrated to the needs of the patient, depending on the premedication given, the type of anaesthesia, and the nature and duration of the surgical procedure.
4. Patient-Controlled Analgesia
Patient-controlled analgesia (PCA) allows patients to assess their own level of pain and consequently titrate the amount of pethidine they require for adequate pain control against sedation and other side effects. Adequate analgesia should be established prior to commencement of PCA.
The dosages and time intervals are preset into a microprocessor-controlled infusion pump. When the patient experiences pain, a button is depressed by the patient and a dose of pethidine is administered intravenously. If the patient should depress the button before the preset time interval (lockout interval) has elapsed, no extra drug is administered. For adults, demand doses, of 5 mg to a maximum of 20 mg pethidine have been given via PCA using a lockout interval of 6 to 20 minutes. Along with the self-administered dose of pethidine, some syringe pumps also deliver a background continuous infusion of pethidine at a basal rate. Some PCA pumps allow a maximum dosage over a defined period to be preset in order to avoid patient overdosage.
The demand dosage and lockout interval should be determined according to the patient’s analgesic requirements. Patients receiving a background infusion of pethidine should generally receive a smaller demand dose relative to equivalent patients utilising a demand dose only.
Clinical experience suggests that patients with normal renal function receiving more than 1000 mg/24hrs pethidine are at particular risk of developing pethidine associated neurotoxicity (PAN). Patients receiving over 800 mg/24hrs pethidine should usually be monitored for early signs of norpethidine toxicity (see Warnings).
Note: Pethidine-associated neurotoxicity is dose related, so pethidine should not be used for periods greater than 24 to 36 hours, see Warnings.
Paediatric Dose
1. Children
Analgesia – Intramuscular (preferred) or subcutaneous, 0.5 to 2 mg per kg of body weight, not to exceed 100 mg, every three to four hours as needed.
Preoperative – Intramuscular (preferred) or subcutaneous, 1 to 2 mg per kg of body weight, not to exceed 100 mg, thirty to ninety minutes prior to anaesthesia.
2. Neonates (see also Pharmacology and Use in Pregnancy)
Excretion and metabolism of pethidine in the neonate is reduced compared with adults. Safety has not been established in neonates and due to lack of data, no dosage regimen can be recommended.
Geriatric patients
Dose reduction to half normal adult dose is recommended in geriatric patients (over 70 years).
Liver impairment
Dosage reduction and/or increased dosage intervals are recommended.
Renal impairment
Due to the possibility of accumulation of norpethidine in patients with renal failure, caution should be exercised when pethidine is administered to these patients, especially over prolonged periods of time. Therefore, a decrease in the dose or increase in the dosing interval is recommended (see also
Precautions).
OVERDOSAGE
Symptoms
Opioid analgesic overdosage usually produces central nervous system depression ranging from stupor to a profound coma, respiratory depression which may progress to Cheyne-Stokes respiration and/or cyanosis, cold clammy skin and/or hypothermia, flaccid skeletal muscles, bradycardia and hypotension. In patients
with severe overdosage, particularly following rapid intravenous administration of an opioid, apnoea, circulatory collapse, cardiac arrest, respiratory arrest and death may occur.
Complications such as pneumonia, shock and/or pulmonary oedema may also prove fatal. Although miosis (pupillary constriction) is characteristic of overdosage with morphine derivatives and methadone, mydriasis may occur in terminal narcosis or severe hypoxia. Overdosage of pethidine may produce mydriasis rather than miosis.
Toxic effects of pethidine may be excitatory, especially in patients who have developed tolerance to the depressant effects of the drug. These patients may exhibit dry mouth, increased muscular activity, muscle tremors and twitches, tachycardia, delirium with disorientation, hallucinations and, occasionally, grand mal seizures.
Treatment
In overdosage, if necessary, establish an airway and institute assisted or controlled ventilation
Circulation should be maintained with infusions of plasma or suitable electrolyte solution. If consciousness is impaired and respiration depressed, an opioid antagonist should be administered. Naloxone, a pure antagonist, is now the treatment of choice. Consult naloxone (or nalorphine) product information. Administer IV naloxone (eg. 0.4 mg) which may be repeated at 2 to 3 minute intervals. For children, the initial dose recommended is 0.01 mg/kg naloxone. In neonates, a more rapid and improved antagonism was noted after 0.02 mg/kg was administered. A response should be seen after 2 or 3 doses. Note the duration of action of naloxone is usually shorter than that of pethidine and thus the patient should be carefully observed for signs of CNS depression returning. An opioid antagonist should not be administered in the absence of clinical signs of respiratory or cardiovascular depression.
Note: In an individual physically dependent on opioids, the administration of the usual dose of an opioid antagonist will precipitate an acute withdrawal syndrome. The severity of this syndrome will depend on the degree of physical dependence and the dose of antagonist administered. The use of opioid antagonists in such individuals should be avoided if possible. If an opioid antagonist must be used to treat serious respiratory depression in the physically dependent patient, the antagonist should be administered with extreme care and only 10 to 20% of the usual initial dose administered.
Storage Conditions
Store at a temperature not exceeding 300C. Do not heats sterilize the formulation.
PROTECTED FROM LIGHT.
How Supplied
VERPAT (Pethidine Injection BP) is supplied as 1ml & 2ml glass ampoule in container of 10 respectively.
Manufactured by:

M/s Verve Human careLaboratories.
Plot No-15-A, Pharmcity,
Selaqui Dehradun,
Uttarakhand (India)
www.vervehumancare.com