Morphine Sulphate Tablet Wholesaler Retailer & Distributor in Chennai, Tamilnadu
Morphine Sulphate Tablet Wholesaler Retailer & Distributor in Chennai, Tamilnadu
What is a Patient Information Leaflet and why is it useful?
The Patient Information Leaflet (PIL) is the leaflet included in the pack with a medicine. It is written for patients and gives information about taking or using a medicine. It is possible that the leaflet in your medicine pack may differ from this version because it may have been updated since your medicine was packaged.
![]() Download LeafletView the patient leaflet in PDF format
Download LeafletView the patient leaflet in PDF format
Below is a text only representation of the Patient Information Leaflet. The original can be viewed in PDF format using the link above.
Morphine Sulphate Tablet Wholesaler Retailer & Distributor in Chennai, Tamilnadu
NRx
VERMOR® SR-10/20/30/60 TABLETS Morphine Sulphate Prolonged Release Tablet BP, 10mg, 20mg, 30mg, 60mg
DESCRIPTION Morphine Sulphate Prolonged Release Tablet BP
Chemically, Morphine Sulphate is 7,8-didehydro-4,5α-epoxy-17-methylmorphinan-3,6α-diol sulphate (2:1) (salt) pentahydrate and has the following structural formula: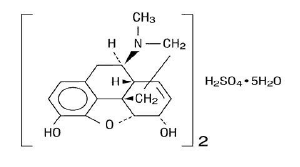
Vermor® SR-10/20/30/60 (Morphine Sulphate Prolonged Release Tablets BP, 10mg, 20mg, 60mg, 60mg) are opiate analgesics supplied in 10mg, 20mg, 30mg, 60mg & 100mg tablet strengths.
PHARMACOLOGY
Morphine is a pure opioid agonist whose principal therapeutic action is analgesia.
Pharmacodynamics
As with all opioids, the minimum effective plasma concentration for analgesia varies widely among patients, especially among patients who have been previously treated with potent agonist opioids. As a result, patients must be treated with individualized titration of dosage to the desired effect. The minimum effective analgesic concentration of morphine for any individual patient may increase over time due to an increase in pain, the development of new pain syndrome and/or the development of analgesic tolerance.
PHARMACOKINETICS
Morphine is released from Morphine Sulphate Prolonged Release Tablets somewhat more slowly than from immediate-release oral preparations. Following oral administration of a given dose of morphine, the amount ultimately absorbed is essentially the same whether the source is Morphine Sulphate Prolonged Release Tablets or an immediate-release formulation. Because of pre-systemic elimination (i.e., metabolism in the gut wall and liver) only about 40% of the administered dose reaches the central compartment.
Absorption
Following the administration of immediate-release oral morphine products, approximately fifty percent of the morphine that will reach the central compartment intact reaches it within 30 minutes. Following the administration of an equal amount of Morphine Sulphate Prolonged Release Tablets to normal volunteers, however, this extent of absorption occurs, on average, after 1.5 hours.
Distribution
The volume of distribution (Vd) for morphine is approximately 4 liters per kilogram. Once absorbed, morphine is distributed to skeletal muscle, kidneys, liver, intestinal tract, lungs, spleen, and brain. Morphine also crosses the placental membranes and has been found in breast milk.
Metabolism
Although a small fraction (less than 5%) of morphine is demethylated, for all practical purposes, virtually all morphine is converted to the 3- and 6- (M3G and M6G) glucuronide metabolites. M3G is present in the highest plasma concentration following oral administration and possesses no significant analgesic activity. M6G, while possessing analgesic activity, is present in the plasma in low concentrations.
Excretion
The elimination of morphine occurs primarily as renal excretion of morphine-3- glucuronide and its terminal elimination half-life after intravenous administration is normally 2 to 4 hours. In some studies involving longer periods of plasma sampling, a longer terminal half-life of about 15 hours was reported. A small amount of the glucuronide conjugate is excreted in the bile, and there is some minor enterohepatic recycling. As with any drug, caution should be taken to guard against unanticipated accumulation if renal and/or hepatic function is seriously impaired.
Drug-Drug Interactions
Known drug-drug interactions involving morphine are pharmacodynamic not pharmacokinetic.
INDICATIONS AND USAGE Morphine Sulphate Prolonged Release Tablet BP
Vermor® SR Tablets is a Prolonged Release oral formulation of morphine Sulphate indicated for the management of moderate to severe pain when a continuous, around-the-clock opioid analgesic is needed for an extended period of time.
The Vermor® SR (Morphine Sulphate Prolonged Release Tablets BP) 100 and 120 mg tablet strengths are high dose, Prolonged Release, oral morphine formulations indicated for the relief of pain in opioid-tolerant patients only.
Vermor® SR Tablets is not indicated for pain in the immediate postoperative period (the first 12-24 hours following surgery) for patients not previously taking the drug, because its safety in this setting has not been established.
Vermor® SR Tablets is not indicated for pain in the postoperative period if the pain is mild, or not expected to persist for an extended period of time.
Vermor® SR Tablets is only indicated for postoperative use if the patient is already receiving the drug prior to surgery or if the postoperative pain is expected to be moderate to severe and persist for an extended period of time.
CONTRAINDICATIONS
Vermor® SR (Morphine Sulphate Prolonged Release Tablets BP) is contraindicated in patients with known hypersensitivity to morphine or in any situation where opioids are contraindicated. This includes patients with respiratory depression (in the absence of resuscitative equipment or in unmonitored settings), and in patients with acute or severe bronchial asthma or hypercarbia.
Vermor® SR(Morphine Sulphate Prolonged Release Tablets BP) is contraindicated in any patient who has or is suspected of having a paralytic ileus.
WARNINGS Morphine Sulphate Prolonged Release Tablet BP
MORPHINE SULPHATE PROLONGED RELEASE TABLETS ARE TO BE SWALLOWED WHOLE AND ARE NOT TO BE BROKEN, CHEWED, DISSOLVED, OR CRUSHED. TAKING BROKEN, CHEWED, DISSOLVED, OR CRUSHED MORPHINE SULPHATE PROLONGED RELEASE TABLETS LEADS TO RAPID RELEASE AND ABSORPTION OF A POTENTIALLY FATAL DOSE OF MORPHINE.
DOSE OF MORPHINE
Patients should be instructed against use by individuals other than the patient for whom it was prescribed, as such inappropriate use may have severe medical consequences, including death.
GENERAL
Vermor® SR Tablets is an Prolonged Release oral formulation of morphine Sulphate indicated for the management of moderate to severe pain when a continuous, around-the-clock analgesic is needed for an extended period of time. Morphine Sulphate Prolonged Release Tablets do not release morphine continuously over the course of a dosing interval.
The administration of single doses of Morphine Sulphate Prolonged Release Tablets on a q12h dosing schedule will result in higher peak and lower trough plasma levels than those that occur when an identical daily dose of morphine is administered using conventional oral formulations on a q4h regimen. The clinical significance of greater fluctuations in morphine plasma level has not been systematically evaluated.
Selection of patients for treatment with Morphine Sulphate Prolonged Release Tablets should be governed by the same principles that apply to the use of morphine or other potent opioid analgesics. Specifically, the increased risks associated with its use in the following populations should be considered: the elderly or debilitated and those with severe impairment of hepatic, pulmonary, or renal function; myxedema or hypothyroidism; adrenocortical insufficiency (e.g., Addison’s Disease); CNS depression or coma; toxic psychosis; prostatic hypertrophy or urethral stricture; acute alcoholism; delirium tremens; kyphoscoliosis or inability to swallow.
The administration of morphine, like all opioid analgesics, may obscure the diagnosis or clinical course in patients with acute abdominal conditions.
Morphine may aggravate convulsions in patients with convulsive disorders, and all opioids may induce or aggravate seizures in some clinical settings.
Carcinogenicity/Mutagenicity/Impairment of Fertility
Studies of morphine Sulphate in animals to evaluate the drug’s carcinogenic and mutagenic potential or the effect on fertility have not been conducted.
Pregnancy
Teratogenic Effects – CATEGORY C
Adequate animal studies on reproduction have not been performed to determine whether morphine affects fertility in males or females. There are no well-controlled studies in women, but marketing experience does not include any evidence of adverse effects on the fetus following routine (short-term) clinical use of morphine Sulphate products. Although there is no clearly defined risk, such experience cannot exclude the possibility of infrequent or subtle damage to the human fetus.
Morphine Prolonged Release Tablets should be used in pregnant women only if the need for strong opioid analgesia clearly outweighs the potential risk to the fetus. (See also: PRECAUTIONS: Labor and Delivery, and WARNINGS: DRUG ABUSE AND ADDICTION.)
Labor and Delivery
Morphine Sulphate Prolonged Release Tablets is not recommended for use in women during and immediately prior to labor. Occasionally, opioid analgesics may prolong labor through actions which temporarily reduce the strength, duration, and frequency of uterine contractions. However, this effect is not consistent and may be offset by an increased rate of cervical dilatation which tends to shorten labor.
Neonates whose mothers received opioid analgesics during labor should be observed closely for signs of respiratory depression. A specific narcotic antagonist, naloxone, should be available for reversal of narcotic-induced respiratory depression in the neonate.
Neonatal Withdrawal Syndrome
Chronic maternal use of opioids during pregnancy can affect the fetus with subsequent withdrawal symptoms. Neonatal withdrawal syndrome presents as irritability, hyperactivity and abnormal sleep pattern, abnormal crying, tremor, vomiting, diarrhea and subsequent weight loss or failure to gain weight, and may result in death. The onset, duration and severity of neonatal withdrawal syndrome varies based on the drug used, duration of use, the dose of last maternal use, and rate of elimination by the newborn. Use standard care as medically appropriate.
Nursing Mothers
Low levels of morphine have been detected in the breast milk. Withdrawal symptoms can occur in breast-feeding infants when maternal administration of morphine Sulphate is stopped. Ordinarily, nursing should not be undertaken while a patient is receiving Morphine Prolonged release tablets since morphine may be excreted in the milk.
Pediatric Use Morphine Sulphate Prolonged Release Tablet BP
Safety and effectiveness in pediatric patients have not been established.
Morphine Sulphate Prolonged release tablets are not to be chewed, crushed, dissolved or divided for administration.
Geriatric Use Morphine Sulphate Prolonged Release Tablet BP
Clinical studies of Morphine Sulphate Prolonged release tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
The adverse reactions caused by morphine are essentially those observed with other opioid analgesics. They include the following major hazards: respiratory depression, apnea, and to a lesser degree, circulatory depression, respiratory arrest, shock, and cardiac arrest.
Most Frequently Observed Morphine Sulphate Prolonged Release Tablet BP
Constipation, lightheadedness, dizziness, sedation, nausea, vomiting, sweating, dysphoria, and euphoria.
Some of these effects seem to be more prominent in ambulatory patients and in those not experiencing severe pain. Some adverse reactions in ambulatory patients may be alleviated if the patient lies down.
Less Frequently Observed Reactions Morphine Sulphate Prolonged Release Tablet BP
Central Nervous System: Weakness, headache, agitation, tremor, uncoordinated muscle movements, seizure, alterations of mood (nervousness, apprehension, depression, floating feelings), dreams, muscle rigidity, transient hallucinations and disorientation, visual disturbances, insomnia, increased intracranial pressure.
Gastrointestinal: Dry mouth, biliary tract spasm, laryngospasm, anorexia, diarrhea, cramps, taste alteration, constipation, ileus, intestinal obstruction, dyspepsia, increases in hepatic enzymes.
Cardiovascular: Flushing of the face, chills, tachycardia, bradycardia, palpitation, faintness, syncope, hypotension, hypertension.
Genitourinary: Urine retention or hesitance, amenorrhea, reduced libido and/or potency
Dermatologic: Pruritus, urticaria, other skin rashes, edema, diaphoresis
Other: Antidiuretic effect, paresthesia, bronchospasm, muscle tremor, blurred vision, nystagmus, diplopia, miosis, anaphylaxis, malaise, thinking disturbances, vertigo.
OVERDOSAGE Morphine Sulphate Prolonged Release Tablet BP
Acute overdosage with morphine can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, rhabdomyolysis progressing to renal failure, and, sometimes, bradycardia, hypotension and death.
The nature of the Prolonged Release morphine should also be taken into account when treating the overdose. Even in the face of improvement, continued medical monitoring is required because of the possibility of extended effects. Deaths due to overdose may occur with abuse and misuse of Morphine Sulphate Prolonged Release Tablets.
In the treatment of morphine overdosage, primary attention should be given to the re-establishment of a patent airway and institution of assisted or controlled ventilation. Supportive measures (including oxygen, vasopressors) should be employed in the management of circulatory shock and pulmonary edema accompanying overdose as indicated. Cardiac arrest or arrhythmias may require cardiac massage or defibrillation.
The pure opioid antagonists, such as naloxone, are specific antidotes against respiratory depression which results from opioid overdose. Naloxone should be administered intravenously; however, because its duration of action is relatively short, the patient must be carefully monitored until spontaneous respiration is reliably re-established. If the response to naloxone is suboptimal or not sustained, additional naloxone may be administered, as needed, or given by continuous infusion to maintain alertness and respiratory function; however, there is no information available about the cumulative dose of naloxone that may be safely administered.
Opioid antagonists should not be administered in the absence of clinically significant respiratory or circulatory depression secondary to morphine overdose. Such agents should be administered cautiously to persons who are known, or suspected to be physically-dependent on Morphine Sulphate Prolonged Release Tablets. In such cases, an abrupt or complete reversal of opioid effects may precipitate an acute abstinence syndrome.
Note: In an individual physically dependent on opioids, administration of the usual dose of the antagonist will precipitate an acute withdrawal syndrome. The severity of the withdrawal syndrome produced will depend on the degree of physical dependence and the dose of the antagonist administered. Use of an opioid antagonist in such a person should be avoided. If necessary to treat serious respiratory depression in the physically dependent patient, the antagonist should be administered with care and by titration with smaller than usual doses of the antagonist.
DOSAGE AND ADMINISTRATION
VERMOR SR tablets should be used at 12-hourly intervals. The dosage is dependent upon the severity of the pain, the patient’s age and previous history of analgesic requirements.
Adults:
A patient presenting with severe pain, uncontrolled by weaker opioids (e.g. dihydrocodeine) should normally be started on 30 mg 12 hourly. Patients previously on normal release oral morphine should be given the same total daily dose as VERMOR SR tablets but in divided doses at 12-hourly intervals.
Increasing severity of pain will require an increased dosage of the tablets. Higher doses should be made, where possible in 30-50% increments as required. The correct dosage for any individual patient is that which is sufficient to control pain with no, or tolerable, side effects for a full 12 hours.
Patients receiving VERMOR SR tablets in place of parenteral morphine should be given a sufficiently increased dosage to compensate for any reduction in analgesic effects associated with oral administration. Usually such increased requirement is of the order of 100%. In such patients individual dose adjustments are required.
Children:
For children with severe cancer pain, a starting dose in the range of 0.2 to 0.8 mg morphine per kg bodyweight 12 hourly is recommended. Doses should then be titrated as for adults.
Post-operative pain:
VERMOR SR tablets are not recommended in the first 24 hours post-operatively or until normal bowel function has returned; thereafter it is suggested that the following dosage schedule be observed at the physician’s discretion:
(a) VERMOR SR tablets 20 mg 12 hourly to patients under 70 kg
(b) VERMOR SR tablets 30 mg 12 hourly to patients over 70 kg
(c) Elderly – a reduction in dosage may be advisable in the elderly
(d) Children – not recommended
Supplemental parenteral morphine may be given if required but with careful attention to the total dosages of morphine, and bearing in mind the prolonged effects of morphine in this prolonged release formulation.
Method of administration
Route of administration: oral
VERMOR SR tablets should be swallowed whole and not broken, chewed or crushed. The administration of broken, chewed or crushed tablets may lead to a rapid release and absorption of a potentially fatal dose of morphine.
Storage: Store in a cool & dry place.
HOW SUPPLIED
Vermor® SR (Morphine Sulphate Prolonged Release Tablets BP) film-coated tablets in a amber colour Alu/Pvc blister pack of 10 Tablets.
Manufactured by:

M/s Verve Human careLaboratories.
Plot No-15-A, Pharmcity,
Selaqui Dehradun,
Uttarakhand (India)
www.vervehumancare.com