Dobamine® Dobutamine Injection 50mg/ml 5ml Ampoule
Dobamine® Dobutamine Injection IP 50mg/ml 5ml Ampoule Patient Information Leaflet
What is a Patient Information Leaflet and why is it useful?
The Patient Information Leaflet (PIL) is the leaflet included in the pack with a medicine. It is written for patients and gives information about taking or using a medicine. It is possible that the leaflet in your medicine pack may differ from this version because it may have been updated since your medicine was packaged.
Download LeafletView the patient leaflet in PDF format
Below is a text only representation of the Patient Information Leaflet. The original can be viewed in PDF format using the link above.
RX
DOBAMINE
Dobutamine Injection IP, 50mg/ml
DESCRIPTION
Dobutamine Hydrochloride is a 1,2-benzenediol,4-[2-[[3-(4-hydro-xyphenyl)-1-methylpropyl]amino]ethyl]-hydrochloride, (±). It is a synthetic catecholamine.
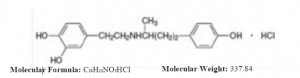
This formulation is supplied in a sterile form for intravenous Infusion use only. Each ml contains: Dobutamine hydrochloride, 50mg; Sodium Bisulfite and sodium metabisulphite and water for injection, q.s. Hydrochloric acid and/or sodium hydroxide may have been added during manufacture to adjust the pH (2.5 to 5.5).
CLINICAL PHARMACOLOGY
PHARMACODYNAMICS
Pharmacotherapeutic group: Adrenergic and dopaminergic agents
ATC Code: C01CA07
Dobutamine is a synthetic, sympathomimetic amine, structurally related to isoproterenol and dopamine, and is administered as racemate. The positive inotropic effect is primarily based on the agonistic effect on cardiac beta1- receptors but also on cardiac alpha1-receptors; which leads to increased contractility with an increase in stroke volume and cardiac output. Dobutamine also has an agonistic effect on peripheral beta2- receptors and to a smaller extent on peripheral alpha2-receptors. In accordance with the harmacological profile, positive chronotropic effects occur as well as effects on the peripheral vascular system. These however, are less pronounced than the effects of other catecholamines. The haemodynamic effects are dose-dependent. The cardiac output increases primarily due to an increase in the stroke volume; an increase in the heart rate is observed particularly with higher dosages. There is a
reduction in left ventricular filling pressure and systemic vascular resistance. With higher doses, there is also a reduction in the pulmonary resistance. Occasionally an insignificant increase of the systemic vascular resistance can be observed. The volume increase due to an increase of the cardiac output is thought to be the reason for the blood pressure elevation.
Dobutamine acts directly, independent from synaptic catecholamine concentrations, does not act at the dopamine receptor site, and – unlike dopamine – has no impact on the release of endogenous noradrenaline (norepinephrine).
There is a decrease of recovery time of sinus node and the A-V conduction time. Dobutamine may cause a tendency towards arrhythmia. When administered non-stop for more than 72 hours, tolerance phenomena were observed. Dobutamine impacts the functions of thrombocytes. Like all other inotropic substances, dobutamine increases myocardial oxygen demand. Via reduction of the pulmonary vascular resistance and the hyperperfusion even of hypoventilated alveolar areas (formation of a pulmonary “Shunt”) a relatively reduced oxygen supply may occur in some cases. The increase in cardiac output and the resulting increase in coronary blood flow usually compensate these effects and cause – compared with other positive inotropic substances – a favourable oxygen supply/demand ratio. Dobutamine is indicated for patients who require positive inotropic support in the treatment of cardiac decompensation due to depressed contractility resulting either from organic heart disease or from cardiac surgical procedures, especially when a low cardiac output is associated with raised pulmonary capillary pressure.
In cases of heart failure accompanied by acute or chronic myocardial ischaemia, administration should be performed in a manner to prevent considerable increase in heart rate or blood pressure; otherwise, articularly in patients with a relatively good ventricular function, increase of ischaemia cannot be excluded.
Paediatric population
Dobutamine also exhibits inotropic effects in children, but the haemodynamic response is somewhat different than that in adults. Although cardiac output increases in children, there is a tendency for systemic vascular resistance and ventricular filling pressure to decrease less and for the heart rate and arterial blood pressure to increase more in children than in adults. Pulmonary wedge pressure may increase during infusion of dobutamine in children 12 months of age or younger. Increases in cardiac output seems to begin at iv infusion rates as low as 1.0 micrograms/kg/minute, increases in systolic blood pressure at 2.5 micrograms/kg/minute, and heart rate changes at 5.5 micrograms/kg/minute. The increase of dobutamine infusion rates from 10 to 20 micrograms/kg/minute usually results in further increases in
cardiac output.
Dobutamine stress echocardiography
Ischaemic diagnostic: Due to the positive inotropic testing and in particular due to the positive chronotropic effects under dobutamine stress, the myocardial oxygen (and substrate) demand increases. With a pre-existing coronary artery stenosis, an insufficient increase of coronary blood flow leads to local hypoperfusion, which can be demonstrated on the echocardiogram in the form of a newly developed myocardial wall motility disorder in the respective segment.
Viability diagnostic: Viable myocardium, which is hypokinetic or akinetic (due to stunning, hibernation) on the echocardiogram, has a contractile functional reserve. This contractile functional reserve is particularly stimulated by the positive inotropic effects during dobutamine stress testing at lower doses (5-20 μg/kg/min). An improvement of the systolic contractility, i.e. increase of wall motility in the respective segment, can be shown on the echocardiogram
PHARMACOKINETICS
Onset of action is 1 – 2 minutes after the start of infusion; during continuing infusion, steady-state plasma levels are only reached after 10 – 12 minutes. Steady-state plasma levels increase dose-dependently linearly to the infusion rate. Half-life is 2 2- 3 minutes, distribution volume is 0.2 l/kg, plasma clearance is not dependent on cardiac output and is 2.4 l/min/m . Dobutamine is mainly metabolised in the tissue and liver. It is mainly metabolised to conjugated glucuronides as well as the pharmacologically inactive 3-O-methyldobutamine. The metabolites are mainly excreted in urine (more than 2/3 of the dose), and to a lesser extent in bile.
Paediatric population
In most paediatric patients, there is a log-linear relationship between plasma dobutamine concentration and hemodynamic response that is consistent with a threshold model.
Dobutamine clearance is consistent with first-order kinetics over the dosage range of 0.5 to 20 micrograms/kg/minute.
Plasma dobutamine concentration can vary as much as two-fold between paediatric patients at the same infusion rate and there is a wide variability in both the plasma dobutamine concentration necessary to initiate a hemodynamic response and the rate of hemodynamic response to increasing plasma concentrations. Therefore, in clinical situations dobutamine infusion rates must be individually titrated.
INDICATION
Dobutamine is indicated for patients who require a positive inotropic support in the treatment of cardiac
decompensation due to depressed contractility.
In cardiogenic shock characterised by heart failure with severe hypotension and in case of septic shock Dobutamine may be useful if added to dopamine in case of disturbed ventricular function, raised filling pressure of the ventricles and raised systemic resistance.
Dobutamine may also be used for detection of myocardial ischaemia and of viable myocardium within the scope of an echocardiographic examination (dobutamine stress echocardiography), if patients cannot undergo a period of exercise or if the exercise yields no information of value.
Paediatric population
Dobutamine is indicated in all paediatric age groups (from neonates to 18 years of age) as inotropic support in low cardiac output hypoperfusion states resulting from decompensated heart failure, following cardiac surgery, cardiomyopathies and in cardiogenic or septic shock.”
DOSAGE AND ADMINISTRATION:
Preparation and Stability: At the same time of administration, Dobamine solution must be further diluted in an IV container to at least a 50 ml solution using one of the following intravenous solutions as diluent: 5% dextrose injection 5% dextrose and 0.45% sodium chloride injection, 5% dextrose and 0.9% sodium chloride injection, 10% dextrose injection, isolyte (R) M with in D5-W, 20% osmitrol (R) in water for injection, 0.9% sodium chloride injection, or sodium lactate injection. Intravenous solutions should be used within 24 hours.
Recommended Dosage: The rate of infusion needed to increase cardiac output usually ranged from 2.5 to 15 mcg/kg/min (see Table 1). On rare occasions, infusion rates up to 40 mcg/kg/min have been required to obtain the desired effect.
Table 1: Dobamine Solution Infusion Rate (ml/kg/min) for Concentrations of 250, 500 and 1000
mcg/ml Infusion Delivery Rate.
Drug Deliv. 250′ 500” 1000”’
Rate (mcg) ml/kg ml/kg ml/kg
Kg/min) /min /min /min
2.5 0.01 0.005 0.0025
5 0.02 0.01 0.005
7.5 0.03 0.015 0.0075
10 0.04 0.02 0.01
12.5 0.05 0.025 0.0125
15 0.06 0.03 0.015
‘250 mcg/ml of diluent
“500 mcg/ml or 250mg/500ml of diluent
“‘1000 mcg/ml or 250mg/250ml of diluent
Rates of Infusion in ml/h for Dobuject solution concentration of 500mcg/ml, 1000mcg/ml and 2000mcg/ml are given in Table 2.
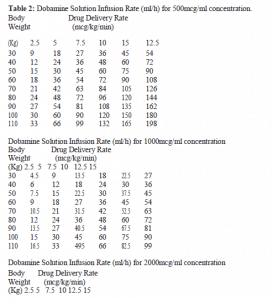
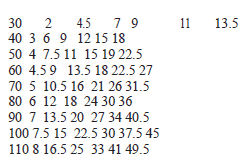
The rate of administration and the duration of therapy should be adjusted according to the patient’s response as determined by heart rate, presence of ectopic activity, blood pressure, urine flow and whenever possible, measurement of central venous or pulmonary wedge pressure and cardiac output. Concentrations up to 5000mcg/ml have been administered to humans (250mg/50ml).
The final volume administered should be determined by the fluid requirements of the patient. Intravenous drug products should be inspected visually and should not be used if particulate matter or discoloration is present.
CONTRAINDICATIONS:
Dobutamine must not be used in the case of: Known hypersensitivity to dobutamine or to any of the excipients, Mechanical obstruction of ventricular filling and/or of outflow, such as pericardial tamponade, constrictive pericarditis, hypertrophic obstructive cardiomyopathy, severe aortic stenosis, Hypovolaemic conditions.
Dobutamine stress echocardiography
Dobutamine must not be used for detection of myocardial ischaemia and of viable myocardium in case of:
Recent myocardial infarction (within the last 30 days),
Unstable angina pectoris,
Stenosis of the main left coronary artery,
Haemodynamically significant outflow obstruction of the left ventricle including hypertrophic obstructive
cardiomyopathy,
Haemodynamically significant cardiac valvular defect,
Severe heart failure (NYHA III or IV),
Predisposition for or documented medical history of clinically significant or chronic arrhythmia, particularly recurrent
persistent ventricular tachycardia,
Significant disturbance in conduction,
Acute pericarditis, myocarditis or endocarditis,
Aortic dissection,
Aortic aneurysm,
Poor sonographic imaging conditions,
Inadequately treated / controlled arterial hypertension,
Obstruction of ventricular filling (constrictive pericarditis, pericardial tamponade),
Hypovolaemia,
Previous experience of hypersensitivity to dobutamine.
Note:
If administering atropine, the respective contraindications have to be observed.
WARNINGS AND PRECAUTIONS
Dobutamine must not be used for the treatment of patients with bronchial asthma who are hypersensitive to sulphites.
A local increase or decrease of coronary blood flow, which may have an impact on the myocardial oxygen demand, has been observed with dobutamine therapy. The clinical characteristics of patients with severe coronary heart disease may deteriorate, in particular if dobutamine therapy is accompanied by a considerable increase in the heart rate and/or blood pressure. Therefore, as with all positive inotropes, the decision to use dobutamine to treat patients with cardiac ischaemia must be made for each case individually.
Due to the risk of arrhythmias and the uncertainty about long term effects on myocardial dysfunction, inotropic agents, such as dobutamine, should be used with caution in the treatment of Acute Heart Failure (AHF).
As alterations in serum potassium level may occur, the potassium level should be monitored.
If dobutamine is administered continuously for more than 72 hours, tolerance phenomena (tachyphylaxis) may occur, requiring dosage increase.
Precipitous decreases in blood pressure (hypotension) have occasionally been described in association with dobutamine therapy. Decreasing the dose or discontinuing the infusion, typically results in rapid return of blood pressure to baseline values, but rarely intervention may be required and reversibility may not be immediate.
Dobutamine may interfere with HPLC determination of chloramphenicol.
Paediatric population
Dobutamine has been administered to children with low-output hypoperfusion states resulting from decompensated heart failure, cardiac surgery, and cardiogenic and septic shock. Some of the haemodynamic effects of dobutamine hydrochloride may be quantitatively or qualitatively different in children as compared to adults.
Increments in heart rate and blood pressure appear to be more frequent and intense in children. Pulmonary wedge pressure may not decrease in children, as it does in adults, or it may actually increase, especially in infants less than one year old. The neonate cardiovascular system has been reported to be less sensitive to dobutamine and hypotensive effect seems to be more often observed in adult patients than in small children. Accordingly, the use of dobutamine in children should be monitored closely, bearing in mind these pharmacodynamic characteristics.
Dobutamine stress echocardiography
Because of possible life-threatening complications, the administration of dobutamine for stress echocardiography should only be undertaken by a physician with sufficient personal experience of the use of dobutamine for this indication.
Dobutamine stress echocardiography must be discontinued if one of the following diagnostic endpoints occurs:
Reaching the age-predicted maximal heart rate [(220-age in years)x0.85],
Systolic blood pressure decrease greater than 20 mmHg,
Blood pressure increase above 220/120 mmHg,
Progressive symptoms (angina pectoris, dyspnoea, dizziness, ataxia),
Progressive arrhythmia (e.g. coupling, ventricular salvos),
Progressive conduction disturbances,
Recently developed wall motility disorders in more than 1 wall segment (16-segment model),
Increase of endsystolic volume,
Development of repolarisation abnormality (due to ischaemia horizontal or down sloping ST segment depression more than 0.2 mV at an interval of 80 (60) ms after the J point compared to baseline, progressive or monophasic ST segment elevation above 0.1 mV in patients without a previous myocardial infarction, Reaching peak dose.
In the event of serious complications (see section 4.8) dobutamine stress echocardiography must be stopped immediately.
After termination of infusion, patients must be monitored until stabilised.
DRUG INTERACTIONS
Via competitive receptor inhibition, the sympathomimetic effect of dobutamine can be reduced by simultaneous administration of a beta receptor blocker. In addition, the alpha agonistic effects may cause peripheral vasoconstriction with a consequent increase in blood pressure.
With simultaneous alpha-receptor blockade, the predominating beta-mimetic effects may cause tachycardia and peripheral vasodilatation.
Simultaneous administration of dobutamine and primarily venous acting vasodilators (e.g. nitrates, sodium nitroprusside) may cause a greater increase of cardiac output as well as a more pronounced decrease of peripheral resistance and ventricular filling pressure than administration of one of the individual substances alone.
Administering dobutamine to diabetic patients may cause increased insulin demand. In diabetic patients insulin levels should be checked when starting dobutamine therapy, changing the rate of infusion and discontinuing the infusion. If necessary the insulin dose must be adjusted as required.
Simultaneous administration of high doses of dobutamine with ACE inhibitors (e.g. captopril) may cause an increase in cardiac output, accompanied by increased myocardial oxygen consumption. Chest pain and rhythm disturbances have been reported in this context.
Dobutamine combined with dopamine causes – depending on the dopamine dosage and in contrast to its sole administration – a more distinct increase of blood pressure as well as a decrease or no change of ventricular filling pressure.
Sodium metabisulphite is a very reactive compound. It must therefore be assumed that thiamine (vitamin B1) coadministered with the preparation is catabolised.
Caution should be exercised when administering dobutamine with inhaled anaesthetics, since concomitant use may increase the excitability of the myocardium and the risk of ventricular extrasystoles.
Dobutamine stress echocardiography
In the case of anti-anginal therapy, in particular heart rate lowering agents like beta-blockers, the ischaemic reaction to stress is less pronounced or may be nonexistent.
Therefore anti-anginal therapy may need to be withheld for 12 hours prior to dobutamine stress echocardiography.
When adding atropine at the highest titration level of dobutamine:
Due to the prolonged duration of the stress echocardiography protocol, the higher total dose of dobutamine and the simultaneous administration of atropine, there is an increased risk of adverse reactions.
STATEMENT ON USAGE DURING PREGNANCYAND LACTATION
As there is no adequate data on the safety of dobutamine in human pregnancy and it is not known whether dobutamine crosses the placenta, dobutamine should not be used during pregnancy unless potential benefits outweigh the potential risks to the foetus and there are no safer therapeutic alternatives.
It is not known, whether dobutamine is excreted in breast milk, so caution should be exercised. If treatment with dobutamine is required for the mother during lactation, breast feeding should be discontinued for the duration of treatment.
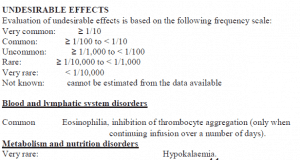


Renal and urinary disorders
Common: Increased urgency at high dosages of infusion.
General disorders and administration site conditions
Common: Fever, phlebitis at the injection site.
Very rare: In case of accidental paravenous infiltration, local inflammation may develop.
Cutaneous necrosis.
Further undesirable effects
Restlessness, nausea, headache, paraesthesia, tremor, urinary urgency, feeling of heat and anxiety, myoclonic spasm.
OVERDOSE AND TREATMENT
Symptoms of overdose
Symptoms are generally caused by excessive stimulation of beta-receptors. Symptoms may include nausea, vomiting, anorexia, tremor, anxiety, palpitations, headache, anginal pain and unspecific chest pain. The positive inotropic and chronotropic cardiac effects may cause hypertension, supraventricular/ventricular arrhythmia and even ventricular fibrillation as well as myocardial ischaemia. Hypotension may occur due to peripheral vasodilatation.
Treatment of overdose
Dobutamine is metabolised rapidly and has a short duration of effect (half-life 2 – 3 minutes).
In case of overdose, administration of dobutamine should be terminated. If necessary, resuscitation procedures must be carried out immediately. Under conditions of intensive care, vital parameters must be monitored and corrected if necessary. Balanced levels of blood gases and serum electrolytes must be maintained.
Severe ventricular arrhythmias can be treated with administration of lidocaine or a beta blocker (e. g. propranolol).
Angina pectoris should be treated with a sublingually administrated nitrate or possibly a short-acting, I.V. beta blocker(e.g. esmolol).
In case of a hypertensive reaction, dose reduction or termination of the infusion is usually sufficient.
With oral administration, the quantity absorbed from the mouth or gastrointestinal tract is unpredictable. In case of accidental oral administration, resorption may be reduced by administration of activated charcoal, which is often more effective than administration of emetics or performing gastric lavage.
The benefit of forced diuresis, peritoneal dialysis, haemodialysis or haemoperfusion via activated charcoal has not been demonstrated for cases of dobutamine overdosage.
Dobutamine stress echocardiography
If applying one of the common dosage schemes, toxic doses are not reached, not even cumulatively.
In case of severe complications during diagnostic administration of dobutamine, the infusion must be terminated at once and sufficient oxygen supply and ventilation must be guaranteed. Treatment of angina pectoris should be performed with an intravenous beta-blocker with a very short-acting effect. Angina pectoris may also be treated with a sublingually administered nitrate, if necessary. Class I and III antiarrhythmics must not be administrated.
STORAGE
Store at a temperature not exceeding 300C. Do not heat -sterilize the formulation. PROTECT FROM LIGHT.
HOW SUPPLIED
DOBAMINE (Dobutamine Injection IP) 50mg/ml. Supplied as: 5ml Amber glass ampoule in container of 5.
Manufactured by:

M/s Verve Human careLaboratories.
Plot No-15-A, Pharmcity,
Selaqui Dehradun,
Uttarakhand (India)
www.vervehumancare.com